
Chemistry, 03.10.2021 01:00 memoryofdale
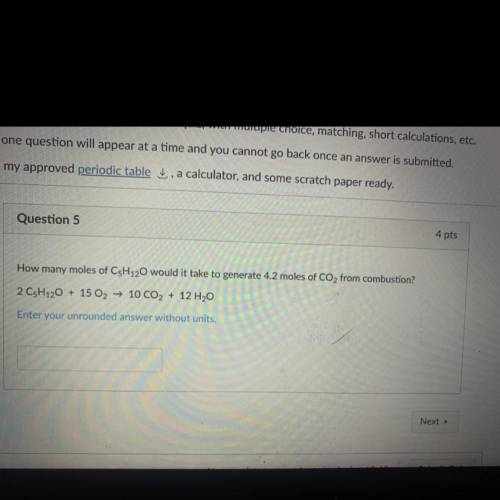
How many moles of C5H120 would it take to generate 4.2 moles of CO2 from combustion?
2 CsH120 + 15 O2 → 10 CO2 + 12 H2O
Enter your unrounded answer without units.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1


Chemistry, 22.06.2019 22:30
Why is it possible for different microorganisms to extract energy not only from carbohydrates and other biological molecules but from a large variety of substances?
Answers: 1
You know the right answer?
How many moles of C5H120 would it take to generate 4.2 moles of CO2 from combustion?
2 CsH120 + 15...
Questions

Mathematics, 11.07.2021 09:20





English, 11.07.2021 09:20



Mathematics, 11.07.2021 09:20

English, 11.07.2021 09:20





Social Studies, 11.07.2021 09:20

Mathematics, 11.07.2021 09:20

Mathematics, 11.07.2021 09:20

Mathematics, 11.07.2021 09:20


Biology, 11.07.2021 09:20



