Chemistry, 03.10.2021 17:40 haileysolis5
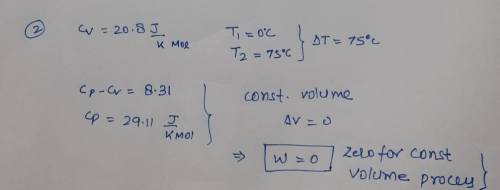
One mole of an ideal gas, with Cv = 20.8 JK-Imol-1 is transformed at
constant volume from 0 °C to 75 °C. Calculate q, w, AU, and AH for
this process. Note that 1 L atm = 101.325 J.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Bohr's model could only explain the spectra of which type of atoms? single atoms with one electron single atoms with more than one electron bonded atoms with one electron bonded atoms with more than one electron
Answers: 2

Chemistry, 22.06.2019 05:30
What royal scientist used the 29th day of frozen vapor to encounter elements for mastering new culinary creations?
Answers: 1

Chemistry, 22.06.2019 06:00
One of the few xenon compounds that form is cesium xenon heptafluoride (csxef7). how many moles of csxef7 can be produced from the reaction of 13.0 mol cesium fluoride with 12.5 mol xenon hexafluoride? csf(s) + xef6(s) csxef7(s)
Answers: 1

Chemistry, 22.06.2019 07:00
The organism shown is a free-living one that is anchored to the bottom of ponds and streams during one stage of its life cycle what is the common name for the group to which this organism belong
Answers: 3
You know the right answer?
One mole of an ideal gas, with Cv = 20.8 JK-Imol-1 is transformed at
constant volume from 0 °C to...
Questions

Chemistry, 25.03.2021 02:20



Mathematics, 25.03.2021 02:20



Mathematics, 25.03.2021 02:20


Mathematics, 25.03.2021 02:20

Mathematics, 25.03.2021 02:20

Mathematics, 25.03.2021 02:20



Social Studies, 25.03.2021 02:20

English, 25.03.2021 02:20



Mathematics, 25.03.2021 02:20

Medicine, 25.03.2021 02:20