
Chemistry, 04.10.2021 01:00 lindseyr190
What mass of silver chloride can be produced from 1.37 L of a 0.138 M solution of silver nitrate? Express your answer with the appropriate units.
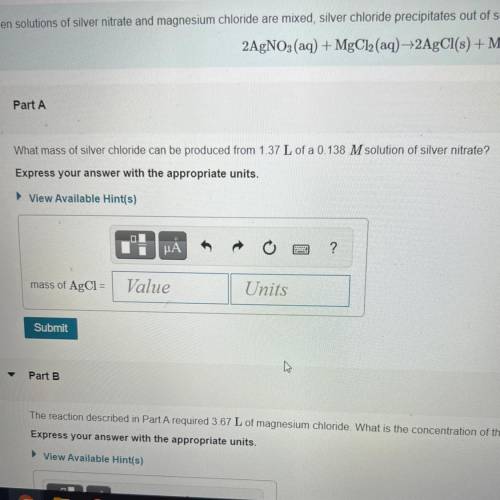

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Janel’s class studied properties of matter and how matter can change. janel decided she would do an experiment mixing baking soda and vinegar.question: describe the properties of baking soda and vinegar, and explain the changes that janel should see when she mixes the two types of matter. •first, identify the physical state of matter of baking soda. describe another property of baking soda. •next, identify the physical state of matter of vinegar. describe another property of vinegar. •then, explain what janel should see when she mixes the baking soda and vinegar. •describe the states of matter of the new materials that are formed. •explain how janel can be certain a change has occurred. me
Answers: 3

Chemistry, 21.06.2019 18:10
Which is true of transition metals when moving from left to right on the periodic table? the d sublevels are not filled across the period. the cation radii become larger across the period. atomic radii increase slightly and then start to decrease. atomic radii decrease slightly and then start to increase. o
Answers: 2

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

You know the right answer?
What mass of silver chloride can be produced from 1.37 L of a 0.138 M solution of silver nitrate? Ex...
Questions

English, 17.07.2019 22:00


Mathematics, 17.07.2019 22:00

Physics, 17.07.2019 22:00


Physics, 17.07.2019 22:00





Chemistry, 17.07.2019 22:00


Chemistry, 17.07.2019 22:00




Mathematics, 17.07.2019 22:00

Chemistry, 17.07.2019 22:00




