
Chemistry, 04.10.2021 14:00 daniel1480
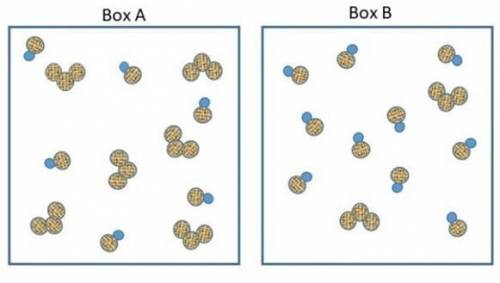
The two pictures shown below represent starting conditions for the following reaction: O3 (g) + NO(g) → O2 (g) + NO2 (g) with a rate law: Rate = k (O3 )(NO)
Which flask will react faster than the other? Determine how much faster the fast one reacts compared to the slow one? Explain your answers in terms of molecular collisions.
How do I find the answer to this? My thinking is that box a will react more since there's equal amounts of reactants but box b will react faster since there's so much NO to collide with the O3 molecules. Thanks.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:00
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2

Chemistry, 23.06.2019 00:00
This statement about matter and its behavior is best classified as a
Answers: 1

Chemistry, 23.06.2019 03:00
The size (radius) of an oxygen molecule is about 2.0 ×10−10m. make a rough estimate of the pressure at which the finite volume of the molecules should cause noticeable deviations from ideal-gas behavior at ordinary temperatures (t= 300k ). assume that deviatons would be noticeable when volume of the gas per molecule equals the volume of the molecule itself.
Answers: 3

Chemistry, 23.06.2019 07:10
1) a light bulb takes in 30 of energy per second. it transfers 3j as use energy. calculate the efficiency. second. it transfers 3j as useful light energy and 27j as heat energy. calculate the efficiency
Answers: 1
You know the right answer?
The two pictures shown below represent starting conditions for the following reaction: O3 (g) + NO(g...
Questions



Mathematics, 18.08.2021 21:00




Advanced Placement (AP), 18.08.2021 21:00

Physics, 18.08.2021 21:00

Mathematics, 18.08.2021 21:00



Biology, 18.08.2021 21:00





English, 18.08.2021 21:00


Social Studies, 18.08.2021 21:00

Biology, 18.08.2021 21:00



