What type of reaction is shown below?
CaCO3(s) + CaO(s) + C02(g)
O A. Combustion
O B....

Chemistry, 08.10.2021 17:40 mmagee2020
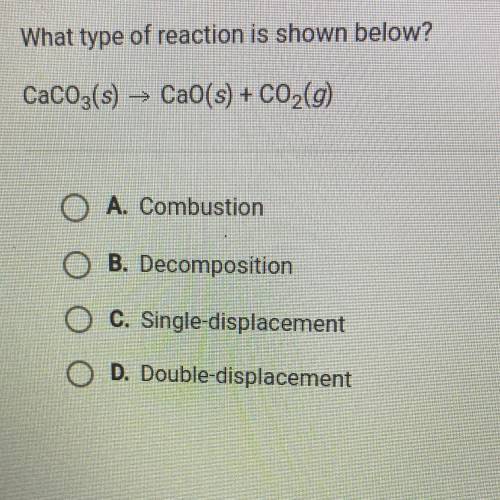
What type of reaction is shown below?
CaCO3(s) + CaO(s) + C02(g)
O A. Combustion
O B. Decomposition
O C. Single-displacement
O D. Double-displacement

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Which statements are true about electrolysis? check all that apply. electrolysis requires an acid be present. electrolysis is described by two half-reactions. electrolysis is not an industrial process. electrolysis results in commercially valuable products. electrolysis involves the transfer of electrons. reduction results in the loss of electrons. oxidation results in the loss of electrons.
Answers: 1

Chemistry, 22.06.2019 05:20
Why does the sun appear to be the brightest star in the sky? a- its apparent brightness is much greater than other stars. b- it burns more gas, making it brighter than any other star. c- it is the largest star in the galaxy, so it is the brightest star. d- its relative distance to earth is closer than the other stars.
Answers: 1

Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

Chemistry, 22.06.2019 11:10
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1
You know the right answer?
Questions


Mathematics, 05.05.2020 14:21

Mathematics, 05.05.2020 14:21


Biology, 05.05.2020 14:21

Social Studies, 05.05.2020 14:21





Biology, 05.05.2020 14:21


Mathematics, 05.05.2020 14:21

Biology, 05.05.2020 14:21

History, 05.05.2020 14:21

Mathematics, 05.05.2020 14:21


Mathematics, 05.05.2020 14:21

Mathematics, 05.05.2020 14:22

History, 05.05.2020 14:22



