Chemistry, 11.10.2021 14:20 Haleysaraya1
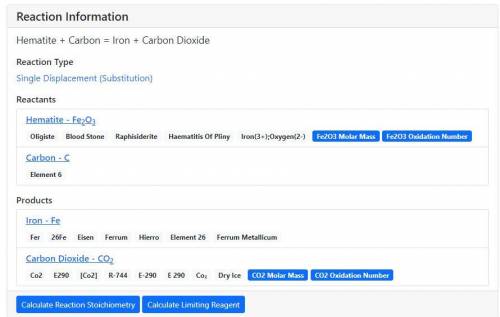
Balance the following chemical equation and determine the number of moles of Fe(s) produced when 8.1E-1
moles of Fe2O3(s) react with excess C(s) to produce Fe(s) and CO2(g).
Fe2O3(s) + C(s) ——)Fe(s) + CO2(g)

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 21:30
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3

Chemistry, 22.06.2019 23:00
Condensation happens when water vapor cools. true or false? ?
Answers: 2

Chemistry, 23.06.2019 03:00
Give a real-world example of an energy transformation that uses two of the following forms of energy: chemical, mechanical, nuclear, gravitational, radiant, electrical, thermal (heat), and/or sound.
Answers: 3
You know the right answer?
Balance the following chemical equation and determine the number of moles of Fe(s) produced when 8.1...
Questions


Chemistry, 22.10.2019 01:00

Biology, 22.10.2019 01:00


Health, 22.10.2019 01:00


History, 22.10.2019 01:00

History, 22.10.2019 01:00

Mathematics, 22.10.2019 01:00


Computers and Technology, 22.10.2019 01:00




Mathematics, 22.10.2019 01:00



English, 22.10.2019 01:00