
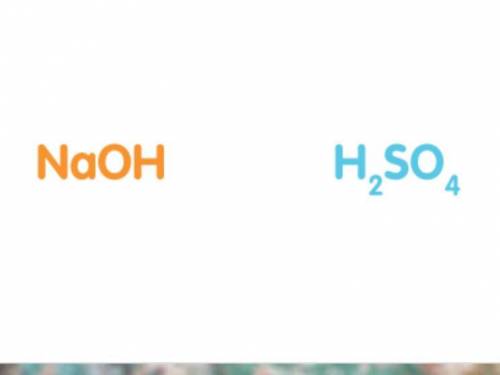
50cm3 of sodium hydroxide solution was titrated against a solution of sulfuric acid. The concentration of the sodium hydroxide solution was 20g/dm3. Work out the concentration of the acid in moles per litre if it took 25cm3 of acid to completely neutralise the alkali. (Hint: It may help to write out a balanced symbol equation for the reaction. The chemical formulas of the acid and alkali are shown below.)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:30
The identities of substances are the same before and after which type of change
Answers: 1

Chemistry, 22.06.2019 17:30
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2

Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1

Chemistry, 22.06.2019 19:30
Which liquid (h2o, h2o + soap, or h2o + salt) has the strongest cohesion and adhesion? (need now plz)
Answers: 1
You know the right answer?
50cm3 of sodium hydroxide solution was titrated against a solution of sulfuric acid. The concentrati...
Questions




Social Studies, 16.02.2022 02:10

Computers and Technology, 16.02.2022 02:10





Mathematics, 16.02.2022 02:10






History, 16.02.2022 02:10


Mathematics, 16.02.2022 02:10





