6
2 points
For the following reaction, what is the theoretical yield in grams of H2O when 05...

Chemistry, 13.10.2021 14:00 noberoger2780
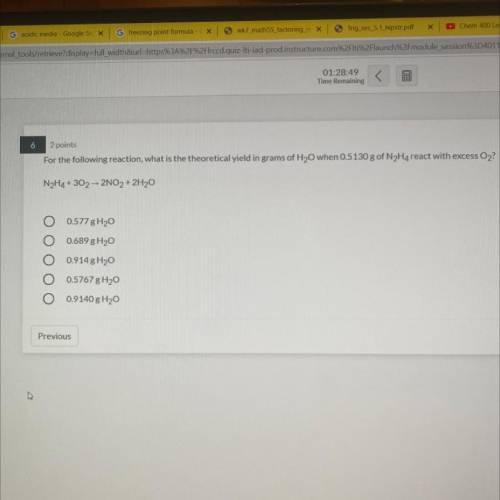
6
2 points
For the following reaction, what is the theoretical yield in grams of H2O when 05130 g of N Ha react with excess O2
NH4+ 30, 2NO, + 2H30
O 0577 8H20
0.689 H2O
ООООО
0914 8H2O
O 0.5767 g 120
O 0.9140 g Hy

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:50
If the mass of the products measured 120g what would the mass of the reactants a. 30g b. 60g c. 120g d. 240g
Answers: 1

Chemistry, 21.06.2019 16:00
Silica, sio2, is formed on silicon as an electrically insulating layer for microelectronic devices. silica is formed when silicon is exposed to o2 gas at an elevated temperature. at 900˚c, it takes 90 minutes for the oxygen to diffuse from the surface to form a 0.06 micron (0.06 x 10-6 m) thick layer of sio2 on
Answers: 1

Chemistry, 22.06.2019 03:30
In saturated organic compounds, all the bonds between carbon atoms are called?
Answers: 1

Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1
You know the right answer?
Questions


Geography, 03.07.2019 17:20

Health, 03.07.2019 17:20

History, 03.07.2019 17:20



World Languages, 03.07.2019 17:20

Physics, 03.07.2019 17:20






Mathematics, 03.07.2019 17:20


Mathematics, 03.07.2019 17:20



Mathematics, 03.07.2019 17:20

English, 03.07.2019 17:20



