
Chemistry, 17.10.2021 14:00 Nayerlin16
Calculate the molality of 44.6% by mass aqeous solution of barrium chloride the molar mass of barrium chloride is 208g/mol
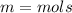

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:30
1. baking powder is a 1: 1 molar mixture of cream of tartar (khc4h4o6) and baking soda (nahco3). a recipe calls for two teaspoons (a total of 8.0 grams) of cream of tartar. how much baking soda must be added for both materials to react completely?
Answers: 2


Chemistry, 23.06.2019 09:00
How many grams of ammonia are produced when 1.0 mole of nitrogen reacts
Answers: 2

Chemistry, 23.06.2019 15:30
Can someone me with these problems? see the attachment, .
Answers: 1
You know the right answer?
Calculate the molality of 44.6% by mass aqeous solution of barrium chloride the molar mass of barriu...
Questions

Spanish, 07.10.2021 08:40


Mathematics, 07.10.2021 08:40


Mathematics, 07.10.2021 08:40

History, 07.10.2021 08:40

Mathematics, 07.10.2021 08:40

Mathematics, 07.10.2021 08:40

Mathematics, 07.10.2021 08:40



Health, 07.10.2021 08:40

History, 07.10.2021 08:40


Health, 07.10.2021 08:40



Mathematics, 07.10.2021 08:40

Geography, 07.10.2021 08:40




