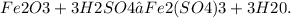Chemistry, 17.10.2021 22:00 kayla114035
Find the mole ratio of H2SO4 and H20 in the equation Fe2O3 + H2SO4 → Fe2(SO4)3 + H20.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2

Chemistry, 22.06.2019 11:50
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3

Chemistry, 22.06.2019 19:50
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3

Chemistry, 22.06.2019 20:00
Acm ruler with main graduations from 1 to 10 from left to right there are 10 secondary graduations between each of the main graduations there is a line that begins. at the left end of the ruler 10 secondary graduations to the left of the “1 main graduation the right end of the line ends on the eighth secondary graduation to the right of 3 how long is the line
Answers: 1
You know the right answer?
Find the mole ratio of H2SO4 and H20 in the equation Fe2O3 + H2SO4 → Fe2(SO4)3 + H20....
Questions



Physics, 29.08.2019 02:30



Mathematics, 29.08.2019 02:30







Mathematics, 29.08.2019 02:30


Computers and Technology, 29.08.2019 02:30



History, 29.08.2019 02:30