
Chemistry, 18.10.2021 08:30 untouchedyannaa
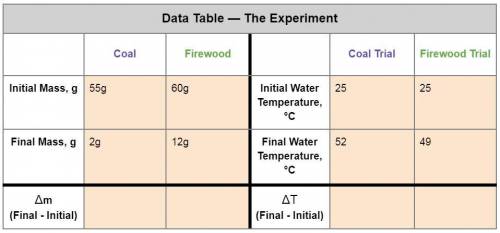
1. Calculate the heat gained by the water when the Firewood was burned.
Equation: q=mc(T f-Ti)
q = heat (J)
m = mass of the water = 50g
c is a constant = 4.184
T f = final temperature
Ti = initial temperature
-
2. Calculate the heat gained by the water when the Coal was burned.
Equation: q=mc(T f-Ti)
q = heat (J)
m = mass of the water = 50g
c is a constant = 4.184
T f = final temperature
Ti = initial temperature

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
The density of an unknown gas at 98°c and 740 mmhg is 2.50 g/l. what is the molar mass of the gas with work showed?
Answers: 1

Chemistry, 22.06.2019 05:30
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1

Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1

Chemistry, 22.06.2019 15:00
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2
You know the right answer?
1. Calculate the heat gained by the water when the Firewood was burned.
Equation: q=mc(T f-Ti)
Questions


Mathematics, 30.12.2019 20:31


Mathematics, 30.12.2019 20:31

Mathematics, 30.12.2019 20:31

Mathematics, 30.12.2019 20:31

Mathematics, 30.12.2019 20:31





English, 30.12.2019 20:31


Mathematics, 30.12.2019 20:31

Mathematics, 30.12.2019 20:31

History, 30.12.2019 20:31

Mathematics, 30.12.2019 20:31

Mathematics, 30.12.2019 20:31





