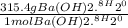WILL GIVE BRAINLIEST AND 20 POINTS! Barium hydroxide, often used to titrate weak organic acids, is obtained as the octahydrate, Ba(OH)2 * 8 H2O. What mass of Ba(OH)2 * 8 H2O would be required to make 500 mL of a solution that is 0.1500 M hydroxide ions? [hint: calculate the molar mass of barium hydroxide octahydrate].

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 22:30
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1

Chemistry, 23.06.2019 00:00
How many moles of water are created if 3 moles of hydrogen react completely with excess oxygen?
Answers: 3

Chemistry, 23.06.2019 10:10
Solid tin exists in two forms: white and gray. for the transformation sn(s, white) → sn(s, gray) the enthalpy change is -2.1 kj/mol and the entropy change is -7.4 j/(mol*k). a. calculate the gibbs free energy change for the conversion of 1.00 mol white tin to gray tin at -30℃. b. will white tin convert spontaneously to gray tin at -30℃? c. at what temperature are white and gray tin thermodynamically equivalent at a pressure of 1 atm?
Answers: 3

You know the right answer?
WILL GIVE BRAINLIEST AND 20 POINTS!
Barium hydroxide, often used to titrate weak organic acids, is...
Questions

Biology, 23.04.2020 01:27




Mathematics, 23.04.2020 01:27








English, 23.04.2020 01:28

Mathematics, 23.04.2020 01:28

Biology, 23.04.2020 01:28

History, 23.04.2020 01:28

Chemistry, 23.04.2020 01:28

History, 23.04.2020 01:28


 ×
× ×
×