The ammonium ion (NH4*, pKa
= 9.25) has a lower pKa than the
methylammonium ion (CH, NHg*, p...

Chemistry, 20.10.2021 09:40 ariloveshorses
The ammonium ion (NH4*, pKa
= 9.25) has a lower pKa than the
methylammonium ion (CH, NHg*, pK. = 10.66). Which is the stronger
base, ammonia (NH.) or methylamine (CHaNH2)? Explain.
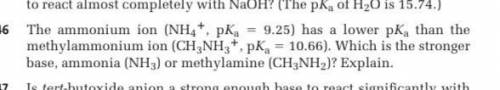

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 08:30
How does the principle of electromagnetism explain the interaction between earth’s magnetic field and the solar wind?
Answers: 1


Chemistry, 22.06.2019 14:30
Amixture that has two or more substances that are spread out evenly is called a. compound b. heterogeneous c. substance d. homogeneous
Answers: 1
You know the right answer?
Questions

Mathematics, 12.02.2021 22:40

Mathematics, 12.02.2021 22:40


Chemistry, 12.02.2021 22:40

Mathematics, 12.02.2021 22:40

Social Studies, 12.02.2021 22:40


Computers and Technology, 12.02.2021 22:40


Mathematics, 12.02.2021 22:40






Mathematics, 12.02.2021 22:40

Mathematics, 12.02.2021 22:40

Mathematics, 12.02.2021 22:40


Mathematics, 12.02.2021 22:40



