O
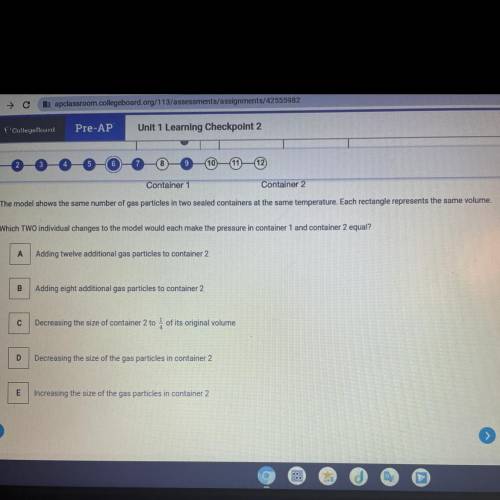
Container 1
Container 2
The model shows the same number of gas particles in two seal...

Chemistry, 20.10.2021 14:00 pleasehelpme666
O
Container 1
Container 2
The model shows the same number of gas particles in two sealed containers at the same temperature. Each rectangle represents the same volume.
Which TWO individual changes to the model would each make the pressure in container 1 and container 2 equal?
A Adding twelve additional gas particles to container 2
B
Adding eight additional gas particles to container 2
с
Decreasing the size of container 2 to 4 of its original volume

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
A6.10 m nacl can be made by adding [x]g of nacl to a container and making the volume of water up to the 1.00 l line
Answers: 1



Chemistry, 23.06.2019 07:30
Which statement is actually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 2
You know the right answer?
Questions

Mathematics, 13.01.2021 17:50



English, 13.01.2021 17:50



Mathematics, 13.01.2021 17:50






Mathematics, 13.01.2021 17:50


English, 13.01.2021 17:50


Biology, 13.01.2021 17:50



Chemistry, 13.01.2021 17:50



