
Chemistry, 25.10.2021 18:00 angelinaavila06
Determine the molar solubility for Cr(OH)3 Ksp = 6.3x10^-31 .
a) Set up the ICE table Cr(OH)3 (s) <-> Cr^3+ (aq) + 3OH^- (aq)
b) Ksp expression
c) Determine molar solubility
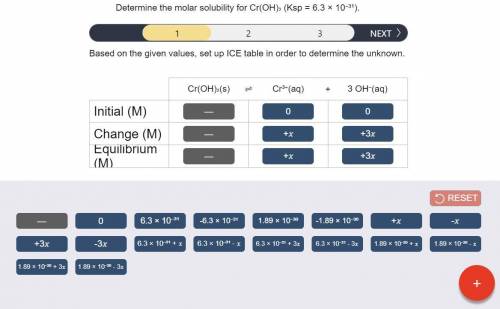
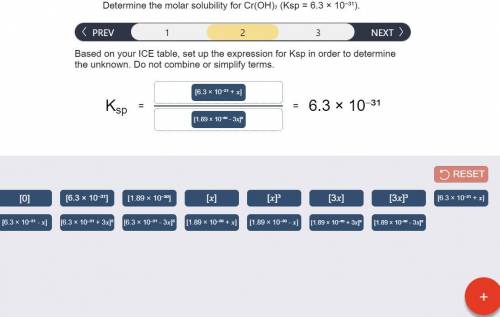
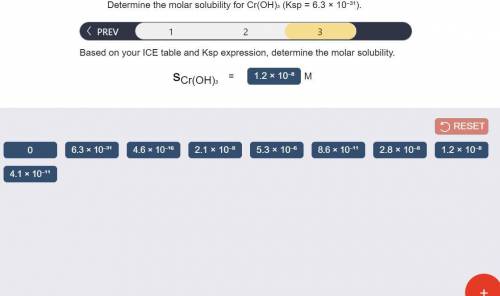

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 04:30
How do i complete this electrolysis of water lab? i’m at home, so i don’t have the materials, and the lab didn’t properly work and was incomplete at school.
Answers: 1

Chemistry, 22.06.2019 08:30
Which metal exist in liquid state and can be cut with knife ?
Answers: 2

You know the right answer?
Determine the molar solubility for Cr(OH)3 Ksp = 6.3x10^-31 .
a) Set up the ICE table Cr(OH)3 (s)...
Questions








English, 24.08.2020 01:01






History, 24.08.2020 01:01

Mathematics, 24.08.2020 01:01

Mathematics, 24.08.2020 01:01







