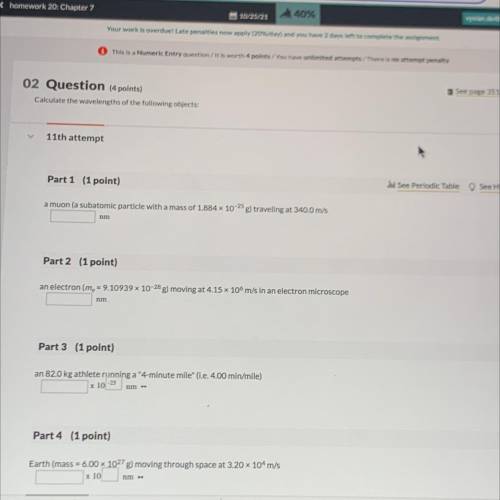
Calculate the wavelengths of the following objects:
Part 1 (1 point)
See Periodic Table See...

Chemistry, 25.10.2021 23:00 chriscook1466
Calculate the wavelengths of the following objects:
Part 1 (1 point)
See Periodic Table See Hint
a muon (a subatomic particle with a mass of 1.884 x 10-25 g) traveling at 340.0 m/s
nm
Part 2 (1 point)
an electron (me = 9.10939 x 10-28 g) moving at 4.15 x 106 m/s in an electron microscope
nm
Part 3 (1 point)
an 82.0 kg athlete running a "4-minute mile" (i. e. 4.00 min/mile)
x 10-25
nm
Part 4 (1 point)
Earth (mass = 6.00 x 10278) moving through space at 3.20 x 10m/s
x 10
nm

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

Chemistry, 22.06.2019 16:00
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2

Chemistry, 22.06.2019 17:00
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2

Chemistry, 22.06.2019 18:30
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1
You know the right answer?
Questions

Mathematics, 12.08.2020 09:01












Chemistry, 12.08.2020 09:01

Mathematics, 12.08.2020 09:01










