
Chemistry, 26.10.2021 14:00 angeladominguezgarci
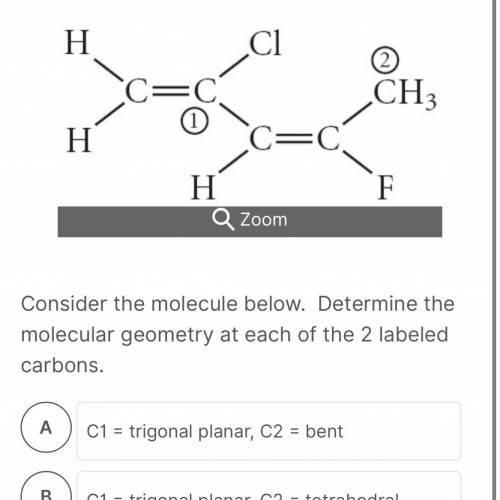
Consider the molecule below. Determine the molecular geometry at each of the 2 labeled carbons. HELP ME PLEASE ASAP
C1 = trigonal planar, C2 = bent
B
C1 = trigonal planar, C2 = tetrahedral
C
C1 = bent, C2 = trigonal planar
D
C1 = tetrahedral, C2 = linear

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2

Chemistry, 22.06.2019 11:00
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1

Chemistry, 22.06.2019 12:00
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2

Chemistry, 22.06.2019 17:20
The small bags of silica gel you often see in a new shoe box are placed there to control humidity. despite its name, silica gel is a solid. it is a chemically inert, highly porous, amorphous form of sio2. because water vapor readily adsorbs onto the surface of silica gel, it acts as a desiccant. despite not knowing mechanistic details of the adsorption of water onto silica gel, from the information provided you should be able to make an educated guess about the thermodynamic characteristics of the process. predict the signs for δg, δh, and δs for the adsorption of water.
Answers: 2
You know the right answer?
Consider the molecule below. Determine the molecular geometry at each of the 2 labeled carbons. HELP...
Questions


Mathematics, 27.07.2019 04:20



Mathematics, 27.07.2019 04:20








History, 27.07.2019 04:20

History, 27.07.2019 04:20

Physics, 27.07.2019 04:20





Geography, 27.07.2019 04:20



