A 50g block of aluminum at -100°C is submerged in 30g of water at 20°C.
Cwater = 4.184 J/gºC
...

Chemistry, 26.10.2021 19:30 lazavionadams81
A 50g block of aluminum at -100°C is submerged in 30g of water at 20°C.
Cwater = 4.184 J/gºC
AH tus water = 334 J/g
Cu = 0.89 J/gºC
What is the final temperature of both substances?
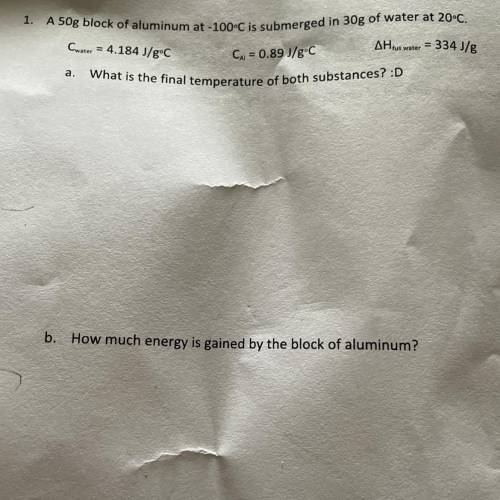

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3


Chemistry, 22.06.2019 16:20
When water dissolves sugar, which process is not involved? o dissociation o hydration o surface area of the solute increases sa
Answers: 1

Chemistry, 22.06.2019 23:00
Movement that is like a t a type of wave that transfers energy where the particles in the medium move in a circle motion while the energy travels left or right. a type of wave that transfers energy where the particles in the medium move perpendicular to the direction in which the energy is traveling. transfers energy from one location to another a type of wave that transfers energy where the particles in the medium move parallel to the direction in which the energy is traveling. movement that is back and forth, like an equal sign = 1. wave 2. parallel movement 3. perpendicular movement 4. transverse wave 5. longitudinal wave 6. surface wave
Answers: 1
You know the right answer?
Questions





Chemistry, 03.08.2019 03:00




Mathematics, 03.08.2019 03:00


Biology, 03.08.2019 03:00


Social Studies, 03.08.2019 03:00

Physics, 03.08.2019 03:00





Mathematics, 03.08.2019 03:00

Advanced Placement (AP), 03.08.2019 03:00



