
Chemistry, 29.10.2021 05:00 TheOneandOnly003
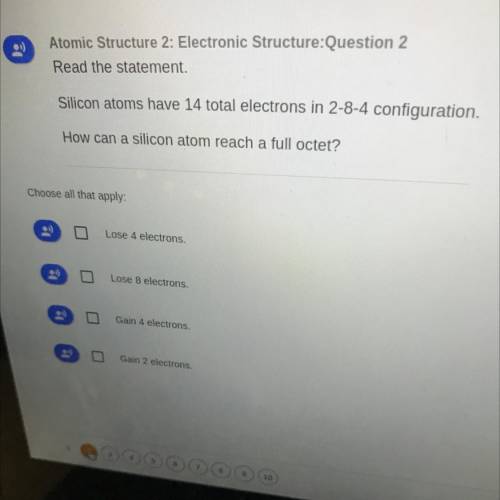
Silicon atoms have 14 total electrons in 2-8-4 configuration. How can a silicon atom reach a full octet?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 23.06.2019 02:00
Butane gas reacts with oxygen gas to give carbon dioxide gas and water vapor (gas). if you mix butane and oxygen in the correct stoichiometric ratio, and if the total pressure of the mixture is 390 mmhg, what is the pressure (in mmhg) of water vapor after the reaction is completed (temperature and volume do not change).
Answers: 2
You know the right answer?
Silicon atoms have 14 total electrons in 2-8-4 configuration.
How can a silicon atom reach a full...
Questions



Mathematics, 21.05.2021 18:50


Engineering, 21.05.2021 18:50

Mathematics, 21.05.2021 18:50


Mathematics, 21.05.2021 18:50



Mathematics, 21.05.2021 18:50

Social Studies, 21.05.2021 18:50

Mathematics, 21.05.2021 18:50


Advanced Placement (AP), 21.05.2021 18:50

Medicine, 21.05.2021 18:50

Spanish, 21.05.2021 18:50

Biology, 21.05.2021 18:50

Biology, 21.05.2021 18:50

Mathematics, 21.05.2021 18:50



