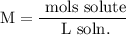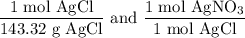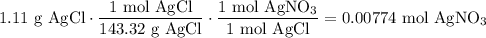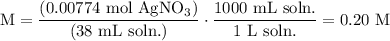Chemistry, 30.10.2021 19:00 jchavez0790
HELPPP PLS IM DESPRATE MAJOR POINTS NEED ASAP
We add excess NaCl solution (58.44 g/mol) to 38 mL of a solution of silver nitrate (AgNO3 169.88 g/mol), to form insoluble solid AgCl. When it has been dried and weighed, the mass of AgCl (143.32 g/mol) is found to be 1.11 grams.
What is the molarity of the original AgNO3 solution? The formula weight of NaNO3 is 85.00 g/mol.
Answer in units of M.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:40
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration?
Answers: 3

Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 22.06.2019 08:00
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1

Chemistry, 22.06.2019 08:40
Which statement can best be concluded from the ideal gas law?
Answers: 2
You know the right answer?
HELPPP PLS IM DESPRATE MAJOR POINTS NEED ASAP
We add excess NaCl solution (58.44 g/mol) to 38 mL o...
Questions


Mathematics, 13.01.2021 03:20

Mathematics, 13.01.2021 03:20




Mathematics, 13.01.2021 03:20


English, 13.01.2021 03:20

History, 13.01.2021 03:20


Mathematics, 13.01.2021 03:20

English, 13.01.2021 03:20



Mathematics, 13.01.2021 03:20


Biology, 13.01.2021 03:20

Mathematics, 13.01.2021 03:20

Chemistry, 13.01.2021 03:20