Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 10:00
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1


Chemistry, 23.06.2019 02:00
Which of the following substances is the most soluble in water? a. sodium chloride b. methane c. bromine d. carbon
Answers: 1
You know the right answer?
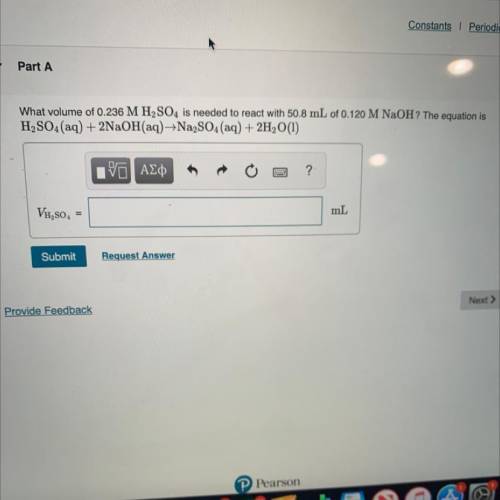
What volume of 0.236 M H2SO4 is needed to react with 50.8 mL of 0.120 M NaOH? The equation is
H2SO...
Questions




English, 03.07.2019 18:30



Mathematics, 03.07.2019 18:30

Mathematics, 03.07.2019 18:30

Chemistry, 03.07.2019 18:30


Chemistry, 03.07.2019 18:30




Biology, 03.07.2019 18:30


Mathematics, 03.07.2019 18:30


Mathematics, 03.07.2019 18:30

History, 03.07.2019 18:30