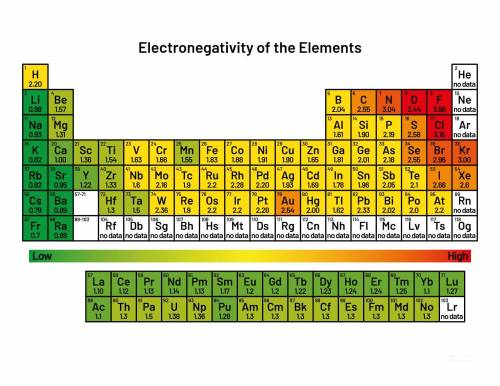
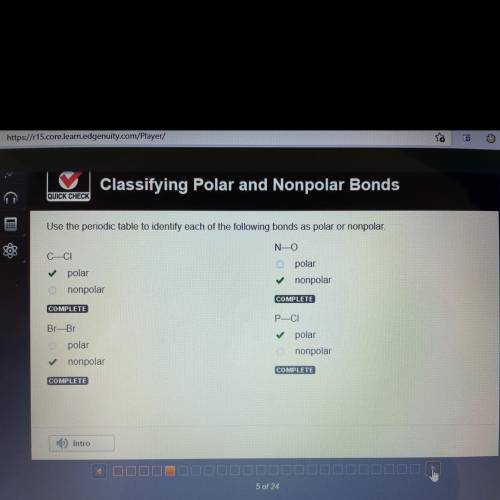
Use the periodic table to identify each of the following bonds as polar or nonpolar.
C — CI
...

Chemistry, 04.11.2021 20:40 megzbrathwaite
Use the periodic table to identify each of the following bonds as polar or nonpolar.
C — CI
= POLAR
Br — Br
= NONPOLAR
N — O
= NONPOLAR
P — CI
= POLAR

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 12:00
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1
You know the right answer?
Questions

English, 25.03.2021 16:00


Mathematics, 25.03.2021 16:00



Mathematics, 25.03.2021 16:00

Mathematics, 25.03.2021 16:00



Mathematics, 25.03.2021 16:00


English, 25.03.2021 16:00

English, 25.03.2021 16:00

Mathematics, 25.03.2021 16:00

Mathematics, 25.03.2021 16:00

English, 25.03.2021 16:00


Arts, 25.03.2021 16:00

English, 25.03.2021 16:00