
Chemistry, 10.11.2021 19:20 daelinrobinson
Use general chemistry rules for significant figures.
1. (12 pts) A cell is constructed to reduce the Ag+ in AgCl(s) to Ag(s) at the cathode when
current is passed through the cell. At the anode, Cu(s) is oxidized to Cu2+.
If current of 0.500 A is passed through the cell for 101 minutes, calculate the mass of
Cu(s) dissolved and mass of Ag(s) deposited. Half-cell reactions:
Cathode: AgCl(s) + e ⇌ Ag(s) + Cl–
Anode: Cu(s) ⇌ Ce2+ + 2e
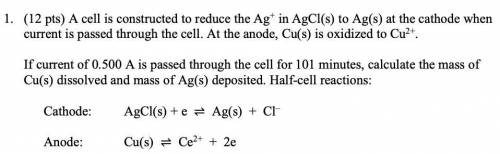

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 2

Chemistry, 22.06.2019 16:30
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2

Chemistry, 22.06.2019 20:20
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1
You know the right answer?
Use general chemistry rules for significant figures.
1. (12 pts) A cell is constructed to reduce t...
Questions

English, 18.01.2022 09:40

Physics, 18.01.2022 09:40


World Languages, 18.01.2022 09:40

Mathematics, 18.01.2022 09:40



History, 18.01.2022 09:40



Biology, 18.01.2022 09:40

Physics, 18.01.2022 09:40

Mathematics, 18.01.2022 09:40

Physics, 18.01.2022 09:40

Chemistry, 18.01.2022 09:40

Mathematics, 18.01.2022 09:40


Mathematics, 18.01.2022 09:50


Computers and Technology, 18.01.2022 09:50



