
Chemistry, 10.11.2021 20:10 theatergeek005
Topics: Fundamentals of electrochemistry; Electrochemical cells
Use general chemistry rules for significant figures.
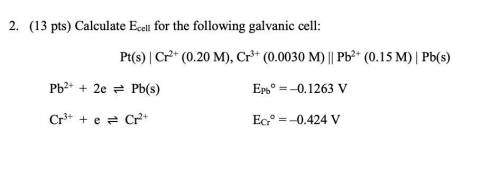
2. (13 pts) Calculate Ecell for the following galvanic cell:
Pt(s) | Cr2+ (0.20 M), Cr3+ (0.0030 M) || Pb2+ (0.15 M) | Pb(s)
Pb2+ + 2e ⇌ Pb(s) EPb° = –0.1263 V
Cr3+ + e ⇌ Cr2+ ECr° = –0.424 V

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 15:00
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2

Chemistry, 22.06.2019 16:30
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1

You know the right answer?
Topics: Fundamentals of electrochemistry; Electrochemical cells
Use general chemistry rules for si...
Questions



Mathematics, 04.02.2021 04:00

Mathematics, 04.02.2021 04:00

Mathematics, 04.02.2021 04:00

Social Studies, 04.02.2021 04:00

Mathematics, 04.02.2021 04:00

Mathematics, 04.02.2021 04:00

Advanced Placement (AP), 04.02.2021 04:00

Mathematics, 04.02.2021 04:00

World Languages, 04.02.2021 04:00

Mathematics, 04.02.2021 04:00






Spanish, 04.02.2021 04:00

Law, 04.02.2021 04:00

Spanish, 04.02.2021 04:00



