
Chemistry, 15.11.2021 02:40 savannadutton8577
In the titration of the weak acid (HA) by a strong base, such as NaOH what is meant by the equivalence point?
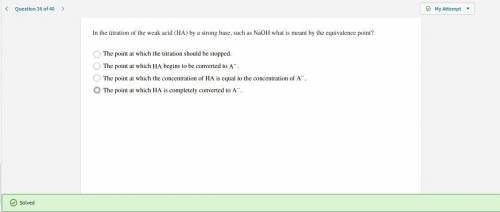

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
A100-watt light bulb radiates energy at a rate of 100 j/s. (the watt, a unit of power or energy over time, is defined as 1 j/s.) if all of the light emitted has a wavelength of 525 nm , how many photons are emitted per second?
Answers: 1

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1

Chemistry, 22.06.2019 18:30
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1

Chemistry, 22.06.2019 20:00
Suppose that some of the compound spilled out of the crucible after it was heated. would that cause the percent by mass of water in the compound determined by the experiment to be too low, too high, or unchanged? briefly explain your answer.
Answers: 1
You know the right answer?
In the titration of the weak acid (HA) by a strong base, such as NaOH what is meant by the equivalen...
Questions





English, 28.02.2021 16:40








Mathematics, 28.02.2021 16:40

Mathematics, 28.02.2021 16:40








