Chemistry, 22.11.2021 23:50 sadeed00974
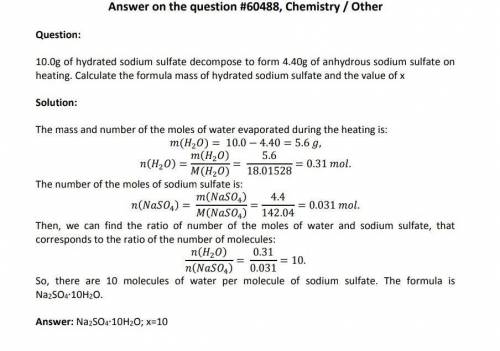
10.00g of hydrated sodium sulfate decomposes to form 4.40g Na(2)SO(4).xH(2)O -> NaSO(4) + xH(2)O of anhydrous sodium sulfate on heating. What’s the formula mass of hydrated sodium sulfate and the value of x? please help i have no clue!
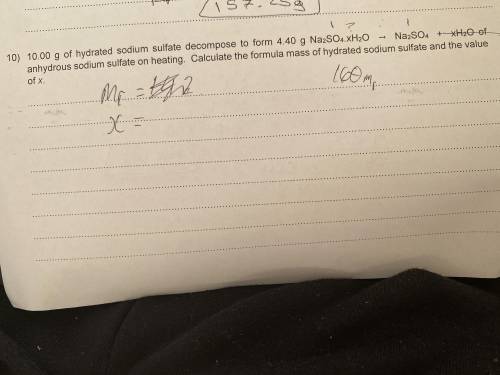

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
State the formula for density in words and mathematical symbols
Answers: 2

Chemistry, 22.06.2019 21:30
The solid xy decomposes into gaseous x and y: xy(s) m x(g) + y(g) kp = 4.1 (at 0 °c) if the reaction is carried out in a 22.4 l container, which initial amounts of x and y will result in the formation of solid xy?
Answers: 1

Chemistry, 23.06.2019 06:10
How can liquids be seperated by density a the liquids are absorbed onto a paper b the liquids are turned into seperate vapors c the liquids are collected as they evaporate d the liquids are allowed to seperate into layers
Answers: 1

You know the right answer?
10.00g of hydrated sodium sulfate decomposes to form 4.40g Na(2)SO(4).xH(2)O -> NaSO(4) + xH(2)O...
Questions


Physics, 12.03.2021 05:00



Mathematics, 12.03.2021 05:00

Mathematics, 12.03.2021 05:00


Computers and Technology, 12.03.2021 05:00


Biology, 12.03.2021 05:00

History, 12.03.2021 05:00


Mathematics, 12.03.2021 05:00

English, 12.03.2021 05:00

Chemistry, 12.03.2021 05:00

Mathematics, 12.03.2021 05:00

Chemistry, 12.03.2021 05:00

History, 12.03.2021 05:00

Biology, 12.03.2021 05:00