
Chemistry, 25.11.2021 14:00 spotty2093
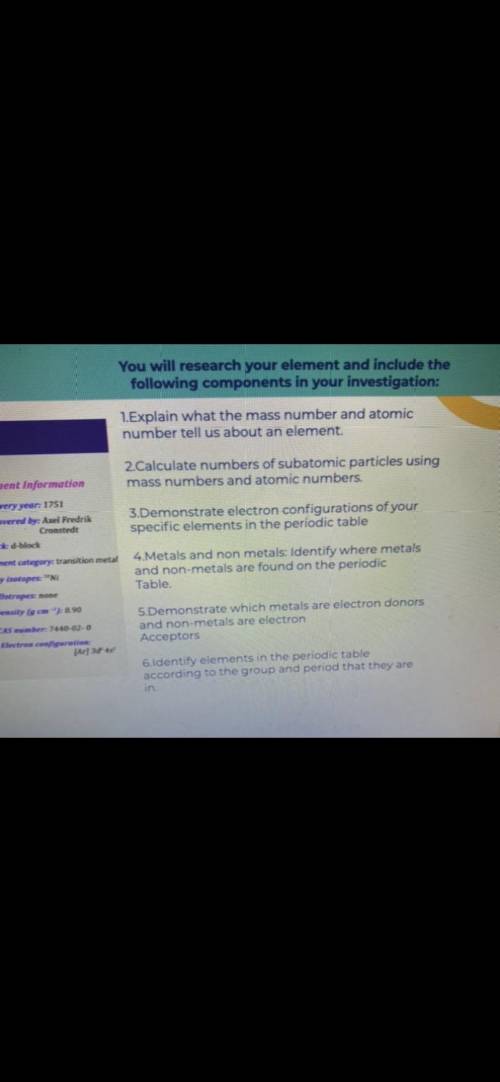
1=Explain what the mass number and atomic number tell us about an element . 2=Calculate numbers of subatomic particles using mass numbers and atomic numbers . 3=Demonstrate electron configurations of your specific elements in the periodic table 4=Metals and non metals : Identify where metals and non - metals are found on the periodic Table . 5=Demonstrate which metals are electron donors and non - metals are electron Acceptors 6=Identify elements in the periodic table according to the group and period that they are

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:10
Which is true of transition metals when moving from left to right on the periodic table? the d sublevels are not filled across the period. the cation radii become larger across the period. atomic radii increase slightly and then start to decrease. atomic radii decrease slightly and then start to increase. o
Answers: 2


Chemistry, 22.06.2019 21:00
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3

Chemistry, 22.06.2019 22:10
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1
You know the right answer?
1=Explain what the mass number and atomic number tell us about an element . 2=Calculate numbers of s...
Questions


Mathematics, 12.08.2020 05:01


Mathematics, 12.08.2020 05:01

Mathematics, 12.08.2020 05:01


Mathematics, 12.08.2020 05:01


Mathematics, 12.08.2020 05:01

Mathematics, 12.08.2020 05:01

Mathematics, 12.08.2020 05:01

Mathematics, 12.08.2020 05:01





Mathematics, 12.08.2020 05:01

Mathematics, 12.08.2020 05:01


Mathematics, 12.08.2020 05:01



