Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2

Chemistry, 22.06.2019 21:30
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1

Chemistry, 23.06.2019 02:20
In a chemical reaction, the final amount of the products is determined by the a. universal gas law b. law of definite proportions c. air pressure d. temperature e. none of the above me
Answers: 2

Chemistry, 23.06.2019 16:00
Which of the following is a reason to make an armature the parent of a creature
Answers: 1
You know the right answer?
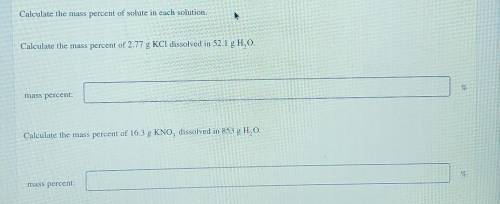
Calculate the mass percent of solute in each solution. Calculate the mass percent of 2.77 g KCl diss...
Questions

Mathematics, 03.12.2021 21:40

Business, 03.12.2021 21:40

Mathematics, 03.12.2021 21:40




Mathematics, 03.12.2021 21:40


Biology, 03.12.2021 21:40

Mathematics, 03.12.2021 21:40


Mathematics, 03.12.2021 21:40

Chemistry, 03.12.2021 21:40

Computers and Technology, 03.12.2021 21:40






Business, 03.12.2021 21:40