
Chemistry, 30.11.2021 08:40 pulliamdylan
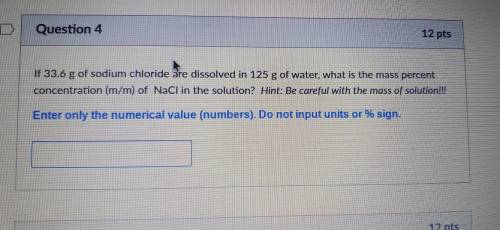
If 33.6 g of sodium chloride are dissolved in 125 g of water, what is the mass percent concentration (m/m) of NaCl in the solution? Hint: Be careful with the mass of solution!!!

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Omg imgonnafailnfiedkla use complete sentences to explain how the mass of hydrogen is conserved during cellular respiration.
Answers: 1

Chemistry, 22.06.2019 01:00
Which statement correctly describes potassium iodide, ki? there is a one-to-one ratio of potassium ions to iodide ions. potassium gains electrons and iodine loses electrons during the reaction. the lattice is held together by potassium anions and iodide cations.
Answers: 1

Chemistry, 22.06.2019 03:30
Each pair of clay balls represents to planetesimals if each plane test molluscum pound of the same material and is separated by the same distance which pair experiences the greatest gravitational attraction
Answers: 2

Chemistry, 22.06.2019 16:00
Sulfuric acid is a polyprotic acid. write balanced chemical equations for the sequence of reactions that sulfuric acid can undergo when it's dissolved in water.
Answers: 2
You know the right answer?
If 33.6 g of sodium chloride are dissolved in 125 g of water, what is the mass percent concentration...
Questions

Social Studies, 06.10.2019 15:30





Spanish, 06.10.2019 15:30

Mathematics, 06.10.2019 15:30




Mathematics, 06.10.2019 15:30


Mathematics, 06.10.2019 15:30

Mathematics, 06.10.2019 15:30

Biology, 06.10.2019 15:30



Chemistry, 06.10.2019 15:30





