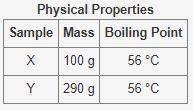
The table below shows some physical properties of two unkown samples
Physical Properties:
Sam...

Chemistry, 01.12.2021 20:40 kakesheco4210
The table below shows some physical properties of two unkown samples
Physical Properties:
Sample 1: X, Mass: 100g, Boiling Point: 56 °C
Sample 2: Y, Mass: 290g, Boiling Point: 56 °C
What answer would best support whether the two samples are of the same substance or not?
A. The two substances are the same because their extensive property is different.
B. The two substances are not the same because their extensive property is the same.
C. The two substances are the same because their intensive property is the same.
D. The two substances are not the same because their intensive property is different.
An image if needed:

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Will mark brainliest 26. which of these statements are true? (3 points) a. gases are compressible b. gases fill their containers completely c. the pressure of a gas is independent of the temperature d. gases have mass e. gases exert pressure f. the pressure of a gas is dependent on the volume g. gas pressure results from the collisions between gas particles h. gases have a definite volume and shape
Answers: 1


Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1

Chemistry, 22.06.2019 23:00
Which organism develops breathing organism develops breathing organs from pharyngeal arches? shark, spider, sea star, sea horse
Answers: 2
You know the right answer?
Questions



English, 03.12.2020 19:20


Spanish, 03.12.2020 19:20

Mathematics, 03.12.2020 19:20

Biology, 03.12.2020 19:20

Mathematics, 03.12.2020 19:20



English, 03.12.2020 19:20

English, 03.12.2020 19:20

Physics, 03.12.2020 19:20


Computers and Technology, 03.12.2020 19:20


Chemistry, 03.12.2020 19:20

Mathematics, 03.12.2020 19:20


Mathematics, 03.12.2020 19:20



