
Chemistry, 02.12.2021 02:30 kiahbryant12
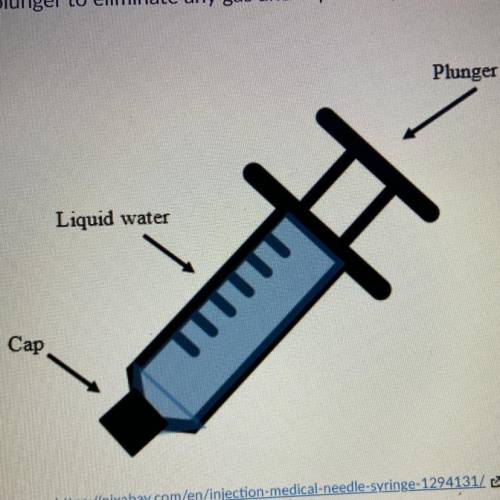
A student is convinced that he is strong enough to compress a liquid. He draws 5 mL of water into a syringe. He pushes the plunger to illuminate any gas in caps the syringe. His set up looks like the image below. With the cap fixed on he pushes the plunger into the syringe with as much force as he can. Which of the following do you expect to happen? 1. He will be able to compress than a liquid because the molecules of water are attracted to one another. 2. He will not be able to compress the liquid because he is not strong enough to break the water bonds. 3. He will not be able to compress the liquid Because the molecules of a liquid to close to because the molecules of a liquid too close together. 4. He will be able to compress than a liquid because there is a lot of space in between the molecules.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
As you watch a surfer ride a wave towards the shoreline, what is the shoreline? a) displacement reference b) reference point c) coordinate plane d) cartesian boundary
Answers: 1

Chemistry, 22.06.2019 07:20
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1

Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1

You know the right answer?
A student is convinced that he is strong enough to compress a liquid. He draws 5 mL of water into a...
Questions

Social Studies, 17.09.2019 06:00

Mathematics, 17.09.2019 06:00


Arts, 17.09.2019 06:00



Mathematics, 17.09.2019 06:00

History, 17.09.2019 06:00


Chemistry, 17.09.2019 06:00



Biology, 17.09.2019 06:00

Mathematics, 17.09.2019 06:00


Social Studies, 17.09.2019 06:00



Mathematics, 17.09.2019 06:00

English, 17.09.2019 06:00



