
Chemistry, 02.12.2021 21:30 Hollywood0122
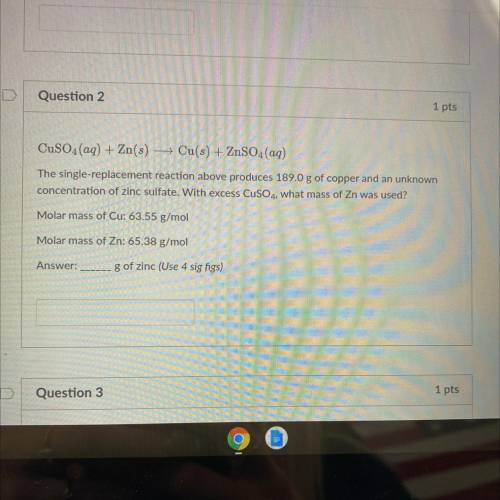
CuSO4(aq) + Zn(s) + Cu(s) + ZnSO4(aq)
The single-replacement reaction above produces 189.0 g of copper and an unknown
concentration of zinc sulfate. With excess CuSO4, what mass of Zn was used?
Molar mass of Cu: 63.55 g/mol
Molar mass of Zn: 65.38 g/mol
g of zinc (Use 4 sig figs)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
If a bottle of olive oil contains 1.4 kg of olive oil, what is the volume, in milliliters ( ml ), of the olive oil?
Answers: 1

Chemistry, 22.06.2019 04:30
The big bang nucleosynthesis theory states that elements were produced in the first few minutes of the big bang while elements have their origins in the interiors of stars, forming much later in the history of the universe.
Answers: 1

Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2

You know the right answer?
CuSO4(aq) + Zn(s) + Cu(s) + ZnSO4(aq)
The single-replacement reaction above produces 189.0 g of co...
Questions

Mathematics, 20.10.2020 09:01

Mathematics, 20.10.2020 09:01

History, 20.10.2020 09:01

English, 20.10.2020 09:01





Mathematics, 20.10.2020 09:01

English, 20.10.2020 09:01


Physics, 20.10.2020 09:01

Mathematics, 20.10.2020 09:01

Mathematics, 20.10.2020 09:01

Law, 20.10.2020 09:01



Mathematics, 20.10.2020 09:01


Geography, 20.10.2020 09:01



