
Chemistry, 03.12.2021 01:50 recon12759
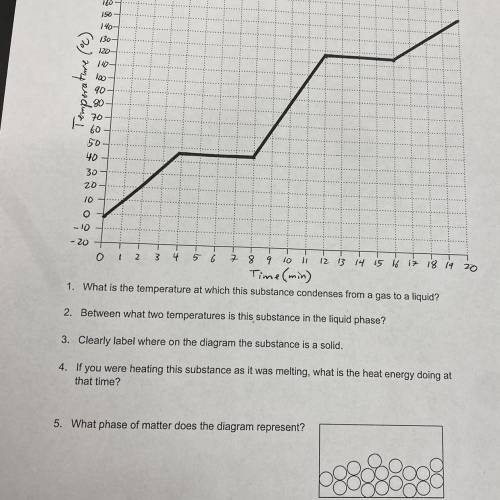
1. What is the temperature at which this substance condenses from a gas to a liquid?
2. Between what two temperatures is this substance in the liquid phase?
3. Clearly label where on the diagram the substance is a solid.
4. If you were heating this substance as it was melting, what is the heat energy doing at that time?
5. What phase of matter does the diagram represent?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:40
Which of the following pressures is equal to 760 mm hg? 2.0 atm 101.3 pa 101,300 kpa 101,300 pa
Answers: 2

Chemistry, 22.06.2019 03:00
Select all that apply. a beta particle: is electromagnetic energy is an electron has zero charge is emitted from the nucleus has a +2 charge has a -1 charge
Answers: 1

Chemistry, 22.06.2019 07:00
How many moles are in 7.2 x 10^23 carbon molecules? (*round to the nearest hundredth and include the unit "mol c" after your number) question 6 options:
Answers: 2

Chemistry, 22.06.2019 09:20
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3
You know the right answer?
1. What is the temperature at which this substance condenses from a gas to a liquid?
2. Between wh...
Questions

History, 27.06.2019 22:00

Mathematics, 27.06.2019 22:00



English, 27.06.2019 22:00

Social Studies, 27.06.2019 22:00


Social Studies, 27.06.2019 22:00

Social Studies, 27.06.2019 22:00

Mathematics, 27.06.2019 22:00

History, 27.06.2019 22:00


Mathematics, 27.06.2019 22:00



History, 27.06.2019 22:00

History, 27.06.2019 22:00

History, 27.06.2019 22:00





