
Chemistry, 03.12.2021 01:50 janelisse199820
The following cations and anions in solution are mixed together, one pair at a time Hg+, K+, Al3+ and I-, S2-, CO3 2- Write a net ionic equation for each precipitate that forms, including states
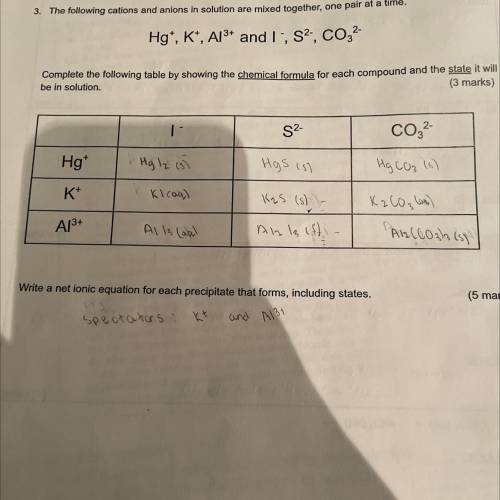

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Select the correct answer. given: 2libr + ba → babr2 + 2li in this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced? a. 1.18 mol b. 2.37 mol c. 4.73 mol d. 16.4 mol e. 32.9 mol
Answers: 2

Chemistry, 22.06.2019 12:30
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3

Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1

Chemistry, 23.06.2019 00:30
What is the chemical formula of magnesium bromide? a. mgbr2 b. mgbr c. mg2br2 d. mg2br
Answers: 3
You know the right answer?
The following cations and anions in solution are mixed together, one pair at a time Hg+, K+, Al3+ an...
Questions


Chemistry, 22.10.2020 19:01





Mathematics, 22.10.2020 19:01


History, 22.10.2020 19:01


Mathematics, 22.10.2020 19:01

Physics, 22.10.2020 19:01



Mathematics, 22.10.2020 19:01


Spanish, 22.10.2020 19:01



Chemistry, 22.10.2020 19:01



