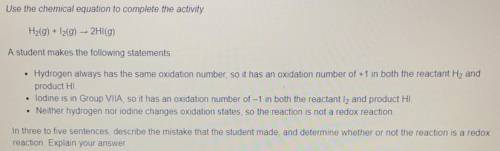
H2(g) + I2(g) -> 2H1(g)

Chemistry, 03.12.2021 06:40 skyhighozzie
Use the chemical equation to complete the
activity.
H2(g) + I2(g) -> 2H1(g)
A student makes the following statements:
- Hydrogen always has the same oxidation number, so it has an oxidation number of +1 in both the reactant H2 and product HI.
- iodine is in Group VIlA, so it has an oxidation number of -1 in both the reactant 12 and product HI.
- Neither hydrogen nor iodine changes oxidation states, so the reaction is not a redox reaction.
In three to five sentences, describe the mistake that the student made, and determine whether or not the reaction is a redox reaction. Explain your answer.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Find the empirical formula of each of the following compounds. given mass or for each element in a sample of the compound 3,611 g ca; 6.389 g c1
Answers: 1

Chemistry, 22.06.2019 07:40
22. a flask containing 450 ml of 0.50 m h2so4 was accidentally knocked to the floor. how many grams of nahco, do you need to put on the spill to neutralize the acid according to the following equation: h2so4(aq)+2 nahcos(aq) na,so(aq) +2 h20()+2 co2(g) d) 38 g a) 2.3 g b) 9.5 g c) 19 g
Answers: 1

Chemistry, 22.06.2019 10:50
Someone offer some answers to this, i will give 98 coins and mark as brainliest! i will put the rest of the lab down in the comments,solutions pre-lab questions: in this lab, you will make fruit drinks with powdered drink mix. complete the pre-lab questions to get the values you need for your drink solutions. calculate the molar mass of powered fruit drink mix, made from sucrose (c12h22o11).using stoichiometry, determine the mass of powdered drink mix needed to make a 1.0 m solution of 100 ml. (hint: use molarity = to find the moles of drink mix, then convert moles to grams using a mole conversion.)what mass of powdered drink mix is needed to make a 0.5 m solution of 100 ml?
Answers: 1

Chemistry, 22.06.2019 11:30
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3
You know the right answer?
Use the chemical equation to complete the
activity.
H2(g) + I2(g) -> 2H1(g)
H2(g) + I2(g) -> 2H1(g)
Questions

Social Studies, 04.08.2019 13:00



Health, 04.08.2019 13:00

Mathematics, 04.08.2019 13:00



Law, 04.08.2019 13:10

English, 04.08.2019 13:10


History, 04.08.2019 13:10





Mathematics, 04.08.2019 13:10



Mathematics, 04.08.2019 13:10

History, 04.08.2019 13:10



