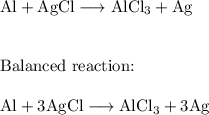Chemistry, 04.12.2021 01:20 kenzierosa
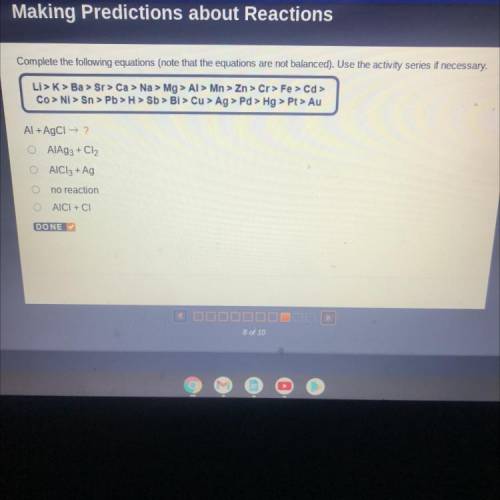
Complete the following equations (note that the equations are not balanced). Use the activity series if necessary.
Li > K> Ba > Sr> Ca > Na > Mg > Al> Mn > Zn > Cr> Fe > Cd >
Co > Ni > Sn > Pb > H > Sb > Bi > Cu > Ag > Pd > Hg > Pt> Au
Al + AgCl → ?
AIAS3 + Cl2
AlCl3 + Ag
no reaction
AICI + CI
DONE

Answers: 1


Another question on Chemistry

Chemistry, 20.06.2019 18:04
What is the smallest component or the most basic building block of any element ? a. an atom, b.a compound c.gas d.element
Answers: 1

Chemistry, 21.06.2019 14:40
What type of solution is formed if 10 g of kclo3 are dissolved in 100g of water at 30
Answers: 2


Chemistry, 21.06.2019 22:00
If a plot weight (in g) vs. volume (in ml) for a metal gave the equation y= 13.41x and r^2=0.9981 what is the density of the metal?
Answers: 2
You know the right answer?
Complete the following equations (note that the equations are not balanced). Use the activity series...
Questions

Mathematics, 24.05.2020 06:59

Mathematics, 24.05.2020 06:59



Mathematics, 24.05.2020 06:59

Social Studies, 24.05.2020 06:59








Social Studies, 24.05.2020 06:59

Mathematics, 24.05.2020 06:59

Mathematics, 24.05.2020 06:59

World Languages, 24.05.2020 06:59

Mathematics, 24.05.2020 06:59

Spanish, 24.05.2020 06:59