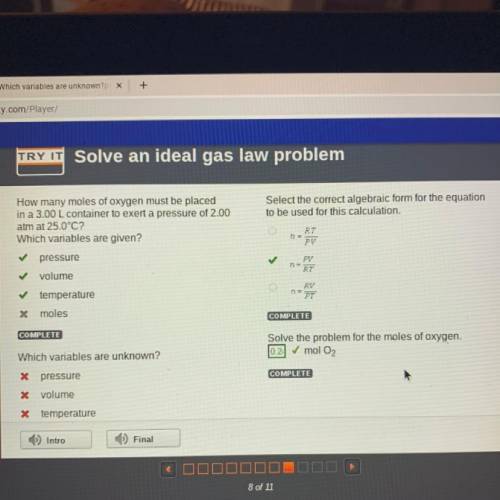
How many moles of oxygen must be placed
in a 3.00 L container to exert a pressure of 2.00
at...

Chemistry, 04.12.2021 01:30 44chandracloutier
How many moles of oxygen must be placed
in a 3.00 L container to exert a pressure of 2.00
atm at 25.0°C?
Which variables are given?
Select the correct algebraic form for the equation
to be used for this calculation.
RT
PV
pressure
✓
PV
RT
✓
volume
22
RV
PT
temperature
* moles
COMPLETE
COMPLETE
Solve the problem for the moles of oxygen.
0.2 mol O2
Which variables are unknown?
X pressure
COMPLETE
X volume
X temperature

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 06:20
If i can still dissolve more sugar into the solution at a certain temperature what would i call that solution
Answers: 3

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 21:00
Which answer tells the reason the earth’s climate is getting warmer? too many animals are becoming extinct. large glaciers are melting in antarctica. the earth is moving closer to the sun. driving cars gives off gases that trap heat in the atmosphere.
Answers: 1
You know the right answer?
Questions


History, 23.08.2019 15:00

Social Studies, 23.08.2019 15:00


Mathematics, 23.08.2019 15:00

Physics, 23.08.2019 15:00




Geography, 23.08.2019 15:00





Biology, 23.08.2019 15:00

Social Studies, 23.08.2019 15:00


Physics, 23.08.2019 15:00

Mathematics, 23.08.2019 15:00

Mathematics, 23.08.2019 15:00



