
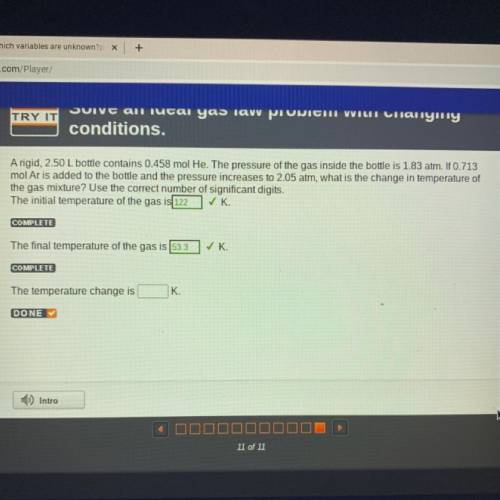
A rigid, 2.50 L bottle contains 0.458 mol He. The pressure of the gas inside the bottle is 1.83 atm. If 0.713
mol Ar is added to the bottle and the pressure increases to 2.05 atm, what is the change in temperature of
the gas mixture? Use the correct number of significant digits.
The initial temperature of the gas is 122 ✓K
COMPLETE
The final temperature of the gas is 53.3
✓ K.
COMPLETE
The temperature change is
K.
DONE

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:40
During which time interval does the object travel approximately 10 meters
Answers: 3

Chemistry, 22.06.2019 01:30
Reaction rate depends on how many molecules are coming into contact with each other with enough energy to react. increasing the temperature of the reactants will increase -
Answers: 3

Chemistry, 22.06.2019 07:00
At 450 mm hg a gas has a volume of 760 l, what is its volume at standard pressure
Answers: 2

Chemistry, 22.06.2019 08:30
Which of the following would be an accurate picture of the earth during the summer time of the northern hemisphere?
Answers: 1
You know the right answer?
A rigid, 2.50 L bottle contains 0.458 mol He. The pressure of the gas inside the bottle is 1.83 atm....
Questions

Business, 21.07.2021 20:00


Mathematics, 21.07.2021 20:00


Mathematics, 21.07.2021 20:00

Chemistry, 21.07.2021 20:00

Mathematics, 21.07.2021 20:00


Mathematics, 21.07.2021 20:00




Physics, 21.07.2021 20:00

English, 21.07.2021 20:00

Mathematics, 21.07.2021 20:00

Mathematics, 21.07.2021 20:00



Spanish, 21.07.2021 20:00



