
Chemistry, 05.12.2021 22:00 Knownothing
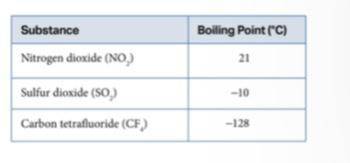
Sulfur dioxide (SO2) and nitrogen dioxide (NO2) both have dipoles, and carbon tetrafluoride (CF4) is nonpolar. All the molecules have relatively similar masses. What could account for the difference in their boiling points?

Answers: 1


Another question on Chemistry




You know the right answer?
Sulfur dioxide (SO2) and nitrogen dioxide (NO2) both have dipoles, and carbon tetrafluoride (CF4) is...
Questions

English, 20.08.2020 22:01


Mathematics, 20.08.2020 22:01

Social Studies, 20.08.2020 22:01




Computers and Technology, 20.08.2020 22:01






Physics, 20.08.2020 22:01


Social Studies, 20.08.2020 22:01



Mathematics, 20.08.2020 22:01



