
Chemistry, 05.12.2021 22:30 emilypzamora11
Q1. How many moles of air are in a 2.8 x 106 L balloon at 20 °C and 750 mmHg of pressure? If the average molar mass of air is 28 g/mol, how many tons of air is this? (1 ton = 2,000 lb)?
Q2. This same balloon is heated from 20 °C to 100 °C keeping the volume and pressure constant. Calculate the new number of tons of gas inside the balloon.
Q3. Calculate the density of the air in the hot air balloon when the air inside is 100 °C in kg/m3.
(Hint: Use the mass you already calculated in question 2!)
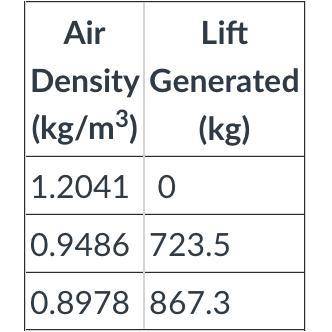
Q4. Use the table attached to determine if the balloon will float at this temperature. (Note: the total mass of the balloon and basket is 723.5 kg. You will need at least that much lift.)

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 07:00
How many moles are in 7.2 x 10^23 carbon molecules? (*round to the nearest hundredth and include the unit "mol c" after your number) question 6 options:
Answers: 2


Chemistry, 23.06.2019 01:00
What type of chemical bond is formed between two atoms of bromine 1. metallic 2. hydrogen 3. ionic 4. covalent
Answers: 1
You know the right answer?
Q1. How many moles of air are in a 2.8 x 106 L balloon at 20 °C and 750 mmHg of pressure? If the ave...
Questions












History, 03.02.2020 23:50






Social Studies, 03.02.2020 23:50

Biology, 03.02.2020 23:50

Mathematics, 03.02.2020 23:50



