
Chemistry, 06.12.2021 03:40 loganparrish2488
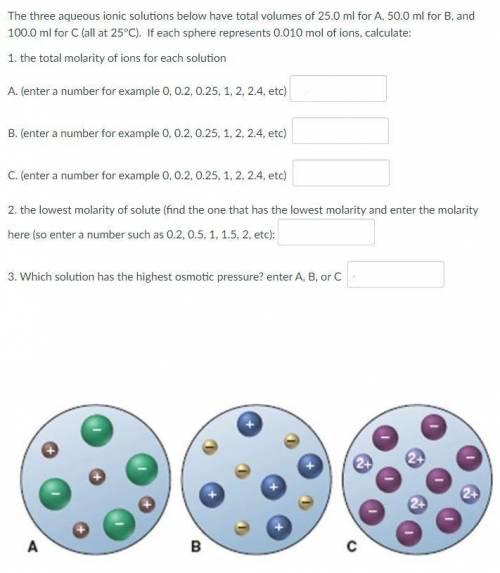
The three aqueous ionic solutions below have total volumes of 25.0 ml for A, 50.0 ml for B, and 100.0 ml for C (all at 25°C). If each sphere represents 0.010 mol of ions, calculate:
1. the total molarity of ions for each solution
2. the lowest molarity of solute
3. Which solution has the highest osmotic pressure?
See the picture attached.
My answers:
1.
A. 3.2
B. 2
C. 1.2
2. 0.4
3. A
Am I right?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 22.06.2019 17:50
Cryolite, na3alf6(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. start this question by first balance the chemical equation.1.) balance the equation: - alo3(s)+naoh(l)+hf(> na3alf6+h2o(g). 2.) if 17.5 kilograms of al2o3(s), 51.4 kilograms of naoh(l), and 51.4 kilograms of hf(g) react completely, how many kilograms of cryolite will be produced? 3.)which reactants will be in excess, (al2o3, naoh, or hf) 4.)what is the total mass of the excess reactants left over after the reaction is complete in kg?
Answers: 2

Chemistry, 23.06.2019 09:30
Sheela and her brother hari were sitting in the living room, watching tv. suddenly hari said that he thinks something is burning in the other room. how did he get the burning smell?
Answers: 3

You know the right answer?
The three aqueous ionic solutions below have total volumes of 25.0 ml for A, 50.0 ml for B, and 100....
Questions

Biology, 23.09.2019 23:30



Mathematics, 23.09.2019 23:30

Biology, 23.09.2019 23:30

Mathematics, 23.09.2019 23:30


History, 23.09.2019 23:30

English, 23.09.2019 23:30

Physics, 23.09.2019 23:30


Mathematics, 23.09.2019 23:30



History, 23.09.2019 23:30

Mathematics, 23.09.2019 23:30

Mathematics, 23.09.2019 23:30

Social Studies, 23.09.2019 23:30


Social Studies, 23.09.2019 23:30



