Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
10. translate each of the following chemical equations into a sentence. a. 2 zns(s) + 3 o2(g) -> 2 zno(s) + 2 so2(g) b. cah2(s) + 2 h2o(l) -> ca(oh)2 (aq) + 2 h2(g)
Answers: 2

Chemistry, 22.06.2019 10:50
How many liters of oxygen gas, at standard temperature and pressure, will react with 35.8 grams of iron metal? 4 fe (s) + 3 o₂ (g) → 2 fe₂o₃ (s)
Answers: 2

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

Chemistry, 22.06.2019 16:50
Which element is least likely to undergo a chemical reaction
Answers: 3
You know the right answer?
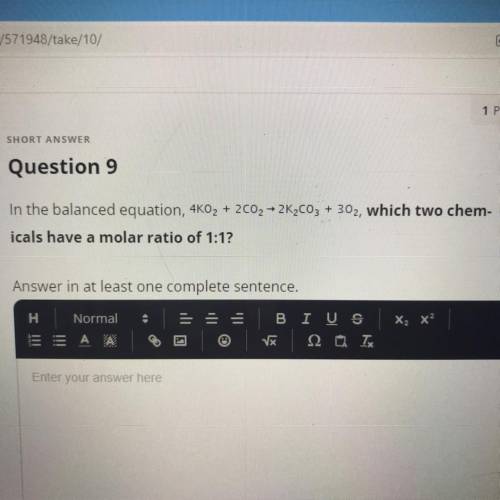
In the balanced equation, 4KO2 + 2002 – 2K2CO3 + 302, which two chem-
icals have a molar ratio of...
Questions



History, 08.07.2019 10:00


Biology, 08.07.2019 10:00

Physics, 08.07.2019 10:00


Mathematics, 08.07.2019 10:00

History, 08.07.2019 10:00



Geography, 08.07.2019 10:00

Social Studies, 08.07.2019 10:00



English, 08.07.2019 10:00

English, 08.07.2019 10:00


Social Studies, 08.07.2019 10:00