D
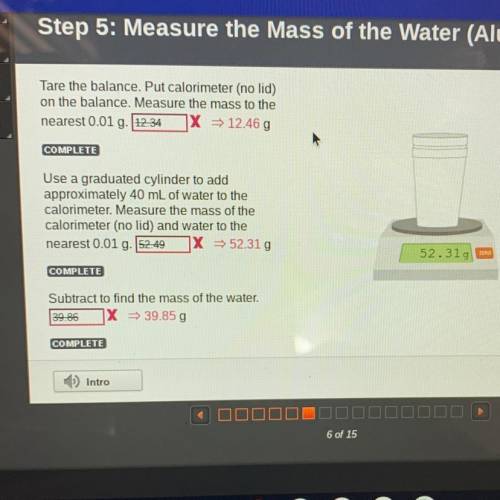
Tare the balance. Put calorimeter (no lid)
on the balance. Measure the mass to the
n...

Chemistry, 08.12.2021 01:00 mexicanvanilla
D
Tare the balance. Put calorimeter (no lid)
on the balance. Measure the mass to the
nearest 0.01 g. 12.34 X = 12.46 g
COMPLETE
Use a graduated cylinder to add
approximately 40 mL of water to the
calorimeter. Measure the mass of the
calorimeter (no lid) and water to the
nearest 0.01 g. 52.49
X = 52.31g
52.319 EEN
COMPLETE
Subtract to find the mass of the water.
39.86
X = 39.85 g
COMPLETE

Answers: 2


Another question on Chemistry


Chemistry, 21.06.2019 18:30
How much energy moves onto the next level, in an energy pyramid
Answers: 1


Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1
You know the right answer?
Questions




Biology, 20.11.2019 09:31

Social Studies, 20.11.2019 09:31

Mathematics, 20.11.2019 09:31

Mathematics, 20.11.2019 09:31


Mathematics, 20.11.2019 09:31

Mathematics, 20.11.2019 09:31





Mathematics, 20.11.2019 09:31

Biology, 20.11.2019 09:31

Biology, 20.11.2019 09:31

Social Studies, 20.11.2019 09:31




