
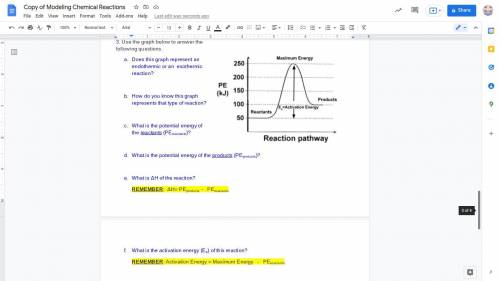
3. Use the graph below to answer the following questions.
Does this graph represent an endothermic or an exothermic reaction?
How do you know this graph represents that type of reaction?
What is the potential energy of the reactants (PEreactants)?
What is the potential energy of the products (PEproducts)?
What is ΔH of the reaction?
REMEMBER: ΔH= PEproducts - PEreactants
What is the activation energy (Ea) of this reaction?
REMEMBER: Activation Energy = Maximum Energy - PEreactants

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:00
The diagram below shows a cell placed in a solution.a cell is shown placed inside a beaker. it is labeled cell. the solution inside the beaker is labeled 40% salt solution and the solution inside the cell is labeled 20% salt solution.only water is allowed to move in and out of the cell. what will most likely happen to the cell? it will expand as water moves out of it. it will shrink as water moves out of it.it will expand as water moves into it. it will shrink as water moves into it.
Answers: 2


Chemistry, 22.06.2019 13:30
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1

Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1
You know the right answer?
3. Use the graph below to answer the following questions.
Does this graph represent an endothermic...
Questions

Arts, 08.11.2019 04:31


English, 08.11.2019 04:31

English, 08.11.2019 04:31

Chemistry, 08.11.2019 04:31

Biology, 08.11.2019 04:31



Mathematics, 08.11.2019 04:31



Mathematics, 08.11.2019 04:31





Mathematics, 08.11.2019 04:31






