
Chemistry, 09.12.2021 06:20 QueenNerdy889
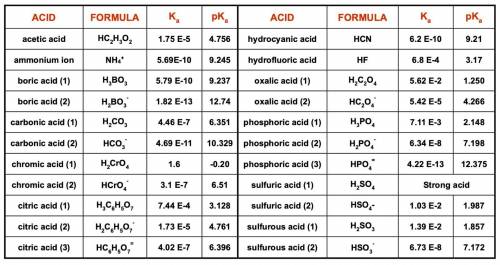
1. List the species present at equilibrium in a solution with the following
composition:
NH4Cl = 0.0200 mol/L NaOH = 0.0430 mol/L
H2SO4 = 0.0150 mol/L NaNO3 = 0.0100 mol/L
2. Write the n equations for n unknowns describing the equilibrium composition of
this system.
3. Make a spreadsheet and use Excel’s Solver function to determine the equilibrium
pH and concentrations of all species in this solution.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1

Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3

Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3

Chemistry, 23.06.2019 00:00
Total the mass on the syringe. record it in the correct row of the data table. kg done click and drag weights to change the pressure. click the syringe to zoom in and see the volume. intro
Answers: 3
You know the right answer?
1. List the species present at equilibrium in a solution with the following
composition:
Questions

Mathematics, 18.04.2021 19:20


Mathematics, 18.04.2021 19:20

Mathematics, 18.04.2021 19:20

Arts, 18.04.2021 19:20

Mathematics, 18.04.2021 19:20

Mathematics, 18.04.2021 19:20

Mathematics, 18.04.2021 19:20



Mathematics, 18.04.2021 19:20


Mathematics, 18.04.2021 19:20



Physics, 18.04.2021 19:20

Arts, 18.04.2021 19:20



Mathematics, 18.04.2021 19:20



