
Chemistry, 10.12.2021 02:00 shadestephen25
iron reacts with oxygen and water to create rust and hydrogen gas as shown in the chemical equation. what mass of rust, to the nearest hundredth gram, is produced in this reaction?
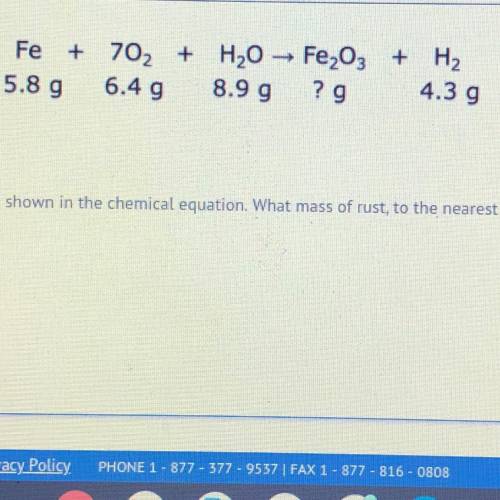

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 15:30
The identities of substances are the same before and after which type of change
Answers: 1

Chemistry, 22.06.2019 21:00
What is the chemical formula for the compound formed between sodium and flour one
Answers: 1

Chemistry, 23.06.2019 00:00
Before it was launched, a helium-filled balloon had a pressure of 201 kpa at a temperature of 27°c. at an altitude of 15,000 m, the pressure had decreased to 2.5 kpa and the temperature had dropped to -14 °c. the volume of the balloon increased to 59.3 m3. what is the original volume of the balloon? 13 m3 0.85 m3 0.077 m3 1.17 m3
Answers: 3

Chemistry, 23.06.2019 00:30
On the periodic table, elements are arranged by which of the following. a. mass numbers. b. increasing atomic number. c. alphabetical order. or d. density
Answers: 1
You know the right answer?
iron reacts with oxygen and water to create rust and hydrogen gas as shown in the chemical equation....
Questions

Mathematics, 14.04.2020 19:55
















History, 14.04.2020 19:56





