
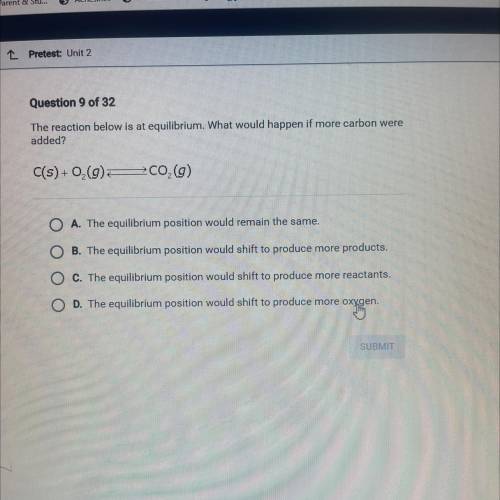
Question 9 of 32
The reaction below is at equilibrium. What would happen if more carbon were
added?
C(s) +0,0 200,0)
A. The equilibrium position would remain the same.
B The equilibrium position would shift to produce more products.
The equilibrium position would shift to produce more reactants.
The equilibrium position would shift to produce more oxygen
SUNT

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 18:00
Which statement best describes the he properties of iconic compounds ?
Answers: 1

Chemistry, 23.06.2019 06:00
Which change will decrease the number of effective collisions during a chemical reaction? a. adding a catalyst b. increasing the surface area c. decreasing the temperature d. increasing the reactant concentrations e. increasing the volume of the reactants
Answers: 2
You know the right answer?
Question 9 of 32
The reaction below is at equilibrium. What would happen if more carbon were
...
...
Questions


Mathematics, 08.04.2021 20:20

Mathematics, 08.04.2021 20:20

English, 08.04.2021 20:20






Computers and Technology, 08.04.2021 20:20

Spanish, 08.04.2021 20:20

Mathematics, 08.04.2021 20:20


English, 08.04.2021 20:20



Mathematics, 08.04.2021 20:20


Biology, 08.04.2021 20:20



