Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:00
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1

Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2

Chemistry, 22.06.2019 13:00
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1

Chemistry, 22.06.2019 23:30
The comparison of the number of atoms in a copper coin the size of a penny with the number of people on earth is made to illustrate which of the following? a. that atoms are indivisible b. that atoms are very small c. that atoms are very large d. that in a copper penny, there is one atom for every person on earth
Answers: 1
You know the right answer?
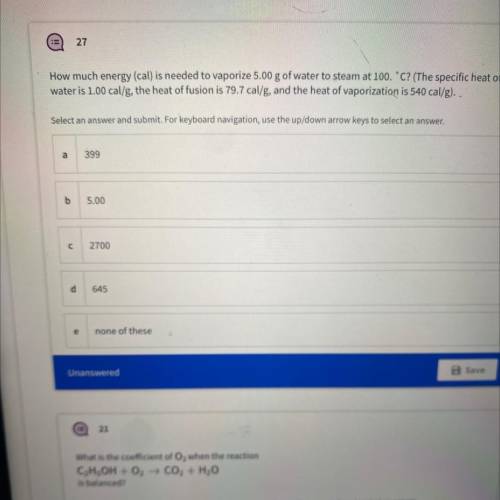
How much energy (cal) is needed to vaporize 5.00 g of water to steam at 100.C? (The specific heat of...
Questions


Geography, 24.06.2019 11:00

Mathematics, 24.06.2019 11:00

Geography, 24.06.2019 11:00

Geography, 24.06.2019 11:00


Geography, 24.06.2019 11:00




Geography, 24.06.2019 11:00


Geography, 24.06.2019 11:00

Mathematics, 24.06.2019 11:00





English, 24.06.2019 11:00

Mathematics, 24.06.2019 11:00