-
-
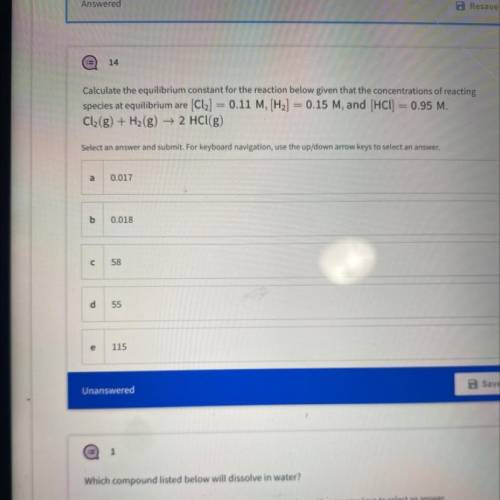
Calculate the equilibrium constant for the reaction below given that the concentrations...

Chemistry, 13.12.2021 06:40 ninigilford
-
-
Calculate the equilibrium constant for the reaction below given that the concentrations of reacting
species at equilibrium are [Cl2] = 0.11 M, [H2] = 0.15 M, and (HCl) = 0.95 M.
Cl2(g) + H2(g) → 2 HCl(g)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2

Chemistry, 22.06.2019 08:30
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1

Chemistry, 23.06.2019 00:50
What is the enthalpy of combustion (per mole) of c4h10 (g)? –2,657.5 kj/mol –5315.0 kj/mol –509.7 kj/mol –254.8 kj/mol
Answers: 1
You know the right answer?
Questions


French, 13.11.2019 05:31

Biology, 13.11.2019 05:31

Mathematics, 13.11.2019 05:31

Mathematics, 13.11.2019 05:31



Mathematics, 13.11.2019 05:31

History, 13.11.2019 05:31



Health, 13.11.2019 05:31

Biology, 13.11.2019 05:31

Physics, 13.11.2019 05:31

Biology, 13.11.2019 05:31

Mathematics, 13.11.2019 05:31

History, 13.11.2019 05:31

Mathematics, 13.11.2019 05:31


Social Studies, 13.11.2019 05:31



