
Chemistry, 13.12.2021 07:10 heybrothwrlogan
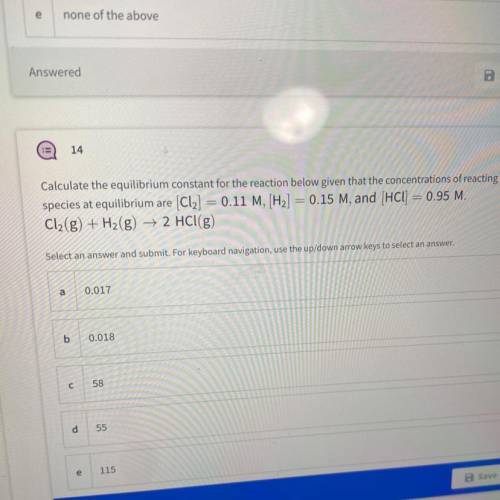
Calculate the equilibrium constant for the reaction below given that the concentrations of reacting
species at equilibrium are (Cl2] = 0.11 M, [H2] = 0.15 M, and (HCl) = 0.95 M.
Cl2(g) + H2(g) → 2 HCl(g)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:30
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2

Chemistry, 23.06.2019 09:00
What sources of error may have contributed to the percent yield not being 100 percent? think about things that may have led to inaccurate measurements or where mass of the product could have been lost if this experiment was conducted in a physical laboratory.
Answers: 2

Chemistry, 23.06.2019 10:20
El amoniaco y el fluor reaccionan para formar tetrafluoruro de dinitrogeno y fluoruro de hidrogeno. segun la reaccion: nh3 + f2 ⇒ n2f4 + hf si reaccionan 5 gramos de amoniaco y 20 gramos de fuor, ¿cuantos gramos de fluoruro de hidrogeno se producen?
Answers: 2

Chemistry, 23.06.2019 11:50
Achemist needs to prepare a buffer solution of ph 8.80. what molarity of nh3 (pkb = 4.75) is required to produce the buffer solution if the (nh4)2so4 in the solution is 1.8 m?
Answers: 1
You know the right answer?
Calculate the equilibrium constant for the reaction below given that the concentrations of reacting...
Questions




Social Studies, 08.10.2021 14:00



Mathematics, 08.10.2021 14:00

Computers and Technology, 08.10.2021 14:00








Physics, 08.10.2021 14:00

English, 08.10.2021 14:00

Mathematics, 08.10.2021 14:00

Computers and Technology, 08.10.2021 14:00

Computers and Technology, 08.10.2021 14:00



